| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2018-03-21 18:00:09 UTC |
|---|
| Update Date | 2018-03-21 18:07:51 UTC |
|---|
| Accession Number | T3D5002 |
|---|
| Identification |
|---|
| Common Name | L-Dopachrome |
|---|
| Class | Small Molecule |
|---|
| Description | Dopachrome is a cyclization product of L-DOPA and is an intermediate in the biosynthesis of melanin. Dopaquinone has an ortho-quinone ring, which is known to be neurotoxic and highly reactive with many other compounds (PMID: 413870). Dopachrome spontaneously gives rise to 5,6-dihydroxyindole (DHI) or it can be enzymatically metabolized by dopachrome tautomerase to give 5,6-dihydroxyindole-2-carboxylic acid (DHICA). DHI and its oxidation products are also toxic to cells. Many Parkinson's patients are treated with L-DOPA. However, long-term treatment with L-DOPA may actually worsen symptoms or may result in neurotic and psychotic symptoms. These may be due to dopachrome and dopaquinone accumulating in the brain of L-DOPA treated patients (PMID: 19131041, PMID: 12373519). |
|---|
| Compound Type | Not Available |
|---|
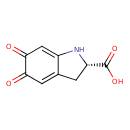
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C9H7NO4 |
|---|
| Average Molecular Mass | 193.158 g/mol |
|---|
| Monoisotopic Mass | 193.038 g/mol |
|---|
| CAS Registry Number | 89762-39-0 |
|---|
| IUPAC Name | (2S)-5,6-dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid |
|---|
| Traditional Name | L-dopachrome |
|---|
| SMILES | OC(=O)[C@@H]1CC2=CC(=O)C(=O)C=C2N1 |
|---|
| InChI Identifier | InChI=1S/C9H7NO4/c11-7-2-4-1-6(9(13)14)10-5(4)3-8(7)12/h2-3,6,10H,1H2,(H,13,14)/t6-/m0/s1 |
|---|
| InChI Key | VJNCICVKUHKIIV-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Indole or derivatives
- Dihydroindole
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine
- Vinylogous amide
- Amino acid
- Ketone
- Cyclic ketone
- Carboxylic acid
- Secondary aliphatic amine
- Enamine
- Monocarboxylic acid or derivatives
- Azacycle
- Secondary amine
- Organoheterocyclic compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0900000000-7d241073b22105a8fd50 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-323f4bcd8528fb467c2b | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ba-7900000000-13c63b10c546db28b452 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-7278b78e7703333d1049 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006w-0900000000-37fa92b16a4a5557156c | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006w-2900000000-a811e0992f3245b7b855 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-becbc589150d5f7b8591 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-96e2a94dd742e6e1937c | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006w-1900000000-1956f467b1b38dab6842 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-ae6d437a06113eb43338 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004m-0900000000-5329f1d5b80270050bbb | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006y-8900000000-bfc43ab0afc8b1b28daf | 2021-09-24 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001430 |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|