| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:59:06 UTC |
|---|
| Update Date | 2014-12-24 20:26:07 UTC |
|---|
| Accession Number | T3D3529 |
|---|
| Identification |
|---|
| Common Name | Polymyxin B Sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Polymyxin B sulfate is a mixture of polymyxins B1 and B2, obtained from Bacillus polymyxa strains. They are basic polypeptides of about eight amino acids and have cationic detergent action on cell membranes. Polymyxin B is used for infections with gram-negative organisms, but may be neurotoxic and nephrotoxic. All gram-positive bacteria, fungi, and the gram-negative cocci, N. gonorrhea and N. menigitidis, are resistant. It is appropriate for treatment of infections of the urinary tract, meninges, and blood stream, caused by susceptible strains of Pseudomonas aeruginosa. |
|---|
| Compound Type | - Amide
- Amine
- Anti-Bacterial Agent
- Drug
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
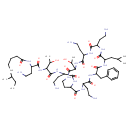
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aerosporin | | Metamyxin | | Polimixina B | | Polomyxin B | | Poly-Rx | | Polyfax | | Polymixin B sulfate | | Polymixin B sulphate | | Polymyxin b | | Polymyxin b sulfate | | Polymyxin B sulfate | | Polymyxin B sulfate salt | | Polymyxin b sulfic acid | | Polymyxin b sulphate | | Polymyxin B sulphate | | Polymyxin B sulphate salt | | Polymyxin b sulphic acid | | Polymyxin-B-Sulfat | | Polymyxine b | | Polymyxine B (sulfate de) | | Polymyxini B sulfas | | Polymyxinum B | | Polyxx |
|
|---|
| Chemical Formula | C56H98N16O13 |
|---|
| Average Molecular Mass | 1203.477 g/mol |
|---|
| Monoisotopic Mass | 1202.750 g/mol |
|---|
| CAS Registry Number | 1405-20-5 |
|---|
| IUPAC Name | N-[3-amino-1-({1-[(3-amino-1-{[6,9,18-tris(2-aminoethyl)-15-benzyl-3-(1-hydroxyethyl)-12-(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl]carbamoyl}propyl)carbamoyl]-2-hydroxypropyl}carbamoyl)propyl]-6-methyloctanamide |
|---|
| Traditional Name | polymyxin B sulfate |
|---|
| SMILES | CCC(C)CCCCC(O)=NC(CCN)C(O)=NC(C(C)O)C(O)=NC(CCN)C(O)=NC1CCN=C(O)C(N=C(O)C(CCN)N=C(O)C(CCN)N=C(O)C(CC(C)C)N=C(O)C(CC2=CC=CC=C2)N=C(O)C(CCN)N=C1O)C(C)O |
|---|
| InChI Identifier | InChI=1/C56H98N16O13/c1-7-32(4)13-11-12-16-44(75)63-36(17-23-57)51(80)72-46(34(6)74)56(85)68-39(20-26-60)48(77)67-41-22-28-62-55(84)45(33(5)73)71-52(81)40(21-27-61)65-47(76)37(18-24-58)66-53(82)42(29-31(2)3)69-54(83)43(30-35-14-9-8-10-15-35)70-49(78)38(19-25-59)64-50(41)79/h8-10,14-15,31-34,36-43,45-46,73-74H,7,11-13,16-30,57-61H2,1-6H3,(H,62,84)(H,63,75)(H,64,79)(H,65,76)(H,66,82)(H,67,77)(H,68,85)(H,69,83)(H,70,78)(H,71,81)(H,72,80) |
|---|
| InChI Key | InChIKey=WQVJHHACXVLGBL-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polypeptides. These are peptides containing ten or more amino acid residues. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic Polymers |
|---|
| Class | Polypeptides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Polypeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polypeptide
- Cyclic alpha peptide
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Monocyclic benzene moiety
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Benzenoid
- Secondary carboxylic acid amide
- Secondary alcohol
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Carbonyl group
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Organic oxygen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 25 mg/mL | | LogP | -4.861 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0900000000-2fc106a8849095860948 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-4910000111-09aa17c0c5e9e1d3a251 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9420000000-50618abf83d40012137d | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-1159-0941000000-9241d7871d46b3a90d91 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bu9-1910000111-2dc8c378fec3170a9d1c | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fu-5921000300-2f55ebc350da3657d7e3 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-2590000000-5b17104b7709c3465894 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fri-1920000010-5f45af1d0f0e081ca1ce | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9420000000-67757bce91a0fadb3044 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-47b6f40976ca4be0e87c | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fdo-6931100016-e4ef6ce119c6e64a028a | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9510000120-71306b33c0302f309ff9 | 2021-10-11 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Eye contact; parenteral (intrathecal, intramuscular injection, intravenous drip). Not absorbed from the normal alimentary tract. |
|---|
| Mechanism of Toxicity | Polymyxin B sulfate has a bactericidal action against almost all gram-negative bacilli except the Proteus group. Polymyxin B sulfate interacts with the lipopolysaccharide of the cytoplasmic outer membrane of Gram-negative bacteria, altering membrane permeability and causing cell death. It does not need to enter the cell. |
|---|
| Metabolism |
Route of Elimination: The drug is excreted slowly by the kidneys. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the treatment of infections of the urinary tract, meninges, and bloodstream caused by susceptible strains of Pseudomonas aeruginosa. It may also be used topically and subconjunctivally in the treatment of infections of the eye caused by susceptible strains of Ps. aeruginosa. (3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Facial flushing, dizziness progressing to ataxia, drowsiness, peripheral paresthesias (circumoral and stocking glove), apnea due to concurrent use of curariform muscle relaxants, other neurotoxic drugs or inadvertent overdosage, and signs of meningeal irritation with intrathecal administration, e.g., fever, headache, stiff neck and increased cell count and protein cerebrospinal fluid. Other reactions occasionally reported: Drug fever, urticarial rash, pain (severe) at intramuscular injection sites, and thrombophlebitis at intravenous injection sites. (3) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00781 |
|---|
| HMDB ID | HMDB14919 |
|---|
| PubChem Compound ID | 4868 |
|---|
| ChEMBL ID | CHEMBL1200626 |
|---|
| ChemSpider ID | 4702 |
|---|
| KEGG ID | C11613 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 506404 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Polymyxin |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Polymyxin_B |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Yuan Z, Tam VH: Polymyxin B: a new strategy for multidrug-resistant Gram-negative organisms. Expert Opin Investig Drugs. 2008 May;17(5):661-8. doi: 10.1517/13543784.17.5.661 . [18447592 ]
- Zavascki AP, Goldani LZ, Li J, Nation RL: Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007 Dec;60(6):1206-15. Epub 2007 Sep 17. [17878146 ]
- Rxlist. Polymyxin-B [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|