| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-18 21:54:32 UTC |
|---|
| Update Date | 2014-12-24 20:23:06 UTC |
|---|
| Accession Number | T3D1064 |
|---|
| Identification |
|---|
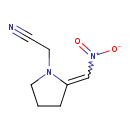
| Common Name | [2-(Nitromethylene)-1-pyrrolidinyl]acetonitrile |
|---|
| Class | Small Molecule |
|---|
| Description | Aromatic heterocycle containing a nitromethylene substituent. Fast acting neurotoxicant, effective both by contact or oral ingestion; they are relatively safe to vertebrates and degrade rapidly in the environment. (1) |
|---|
| Compound Type | - Amine
- Cyanide Compound
- Nitrile
- Nitromethylene
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-[2-(Nitromethylidene)pyrrolidin-1-yl]acetonitrile | | [(2Z)-2-(Nitromethylene)pyrrolidin-1-yl]acetonitrile |
|
|---|
| Chemical Formula | C7H9N3O2 |
|---|
| Average Molecular Mass | 167.165 g/mol |
|---|
| Monoisotopic Mass | 167.069 g/mol |
|---|
| CAS Registry Number | 91417-82-2 |
|---|
| IUPAC Name | 2-[2-(nitromethylidene)pyrrolidin-1-yl]acetonitrile |
|---|
| Traditional Name | 2-[2-(nitromethylidene)pyrrolidin-1-yl]acetonitrile |
|---|
| SMILES | [O-][N+](=O)\C=C1\CCCN1CC#N |
|---|
| InChI Identifier | InChI=1S/C7H9N3O2/c8-3-5-9-4-1-2-7(9)6-10(11)12/h6H,1-2,4-5H2/b7-6- |
|---|
| InChI Key | InChIKey=UUBCVVUIZRGQQU-SREVYHEPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-alkylpyrrolidines. N-alkylpyrrolidines are compounds containing a pyrrolidine moiety that is substituted at the N1-position with an alkyl group. Pyrrolidine is a five-membered saturated aliphatic heterocycle with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolidines |
|---|
| Sub Class | N-alkylpyrrolidines |
|---|
| Direct Parent | N-alkylpyrrolidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-alkylpyrrolidine
- Alpha-aminonitrile
- Organic nitro compound
- Tertiary aliphatic amine
- Tertiary amine
- C-nitro compound
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Nitrile
- Carbonitrile
- Enamine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1900000000-56670ddc768bda48c749 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-8900000000-7a26ce7b0af3045fea0d | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-d6bccb19b75c4e468ff9 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0900000000-0771d7a460bb18c0b3b7 | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-5f1c616cb2029addab3a | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k96-9700000000-54b52dd3b1c0371e6030 | 2019-02-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Acts as a neurotransmitter mimic , having both excitatory and depressant effects, eventually blocking postsynaptic nicotinic receptors. (1) Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (3) |
|---|
| Metabolism | Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (2) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Nitromethylenes are used as pesticides. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Nitromethylenes are neurotoxic. (1) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 2247078 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 1681488 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | [2-(Nitromethylene)-1-pyrrolidinyl]acetonitrile |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- ATSDR - Agency for Toxic Substances and Disease Registry (2006). Toxicological profile for cyanide. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Cyanide poisoning. Last Updated 30 March 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|