| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:03 UTC |
|---|
| Update Date | 2014-12-24 20:21:04 UTC |
|---|
| Accession Number | T3D0084 |
|---|
| Identification |
|---|
| Common Name | Hydrazine |
|---|
| Class | Small Molecule |
|---|
| Description | Being bifunctional, with two amines, hydrazine is a key building block for the preparation of many heterocyclic compounds via condensation with a range of difunctional electrophiles. With 2,4-pentanedione, it condenses to give the 3,5-dimethylpyrazole. In the Einhorn-Brunner reaction hydrazines react with imides to give triazoles. Hydrazine is a convenient reductant because the by-products are typically nitrogen gas and water. Thus, it is used as an antioxidant, an oxygen scavenger, and a corrosion inhibitor in water boilers and heating systems. It is also used to reduce metal salts and oxides to the pure metals in electroless nickel plating and plutonium extraction from nuclear reactor waste. Hydrazine is an inorganic chemical compound with the formula N2H4. It is a colourless liquid with an ammonia-like odor and is derived from the same industrial chemistry processes that manufacture ammonia. However, hydrazine has physical properties that are more similar to those of water. The propanone azine is an intermediate in the Atofina-PCUK synthesis. Direct alkylation of hydrazines with alkyl halides in the presence of base affords alkyl-substituted hydrazines, but the reaction is typically inefficient due to poor control on level of substitution (same as in ordinary amines). The reduction of hydrazones to hydrazines present a clean way to produce 1,1-dialkylated hydrazines. |
|---|
| Compound Type | - Cigarette Toxin
- Food Toxin
- Hydrazine
- Industrial Precursor/Intermediate
- Industrial/Workplace Toxin
- Inorganic Compound
- Metabolite
- Non-Metal
- Organic Compound
- Pollutant
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Amerzine | | Catalyzed hydrazine | | Diamide | | Diamine | | Diazane | | HDZ | | Hydrazin | | Hydrazine (anhydrous) | | Hydrazine base | | Hydrazine solution | | Hydrazines | | Levoxine | | Nitrogen hydride | | Oxytreat 35 | | Scav-ox II | | Zerox |
|
|---|
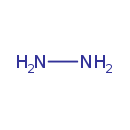
| Chemical Formula | H4N2 |
|---|
| Average Molecular Mass | 32.045 g/mol |
|---|
| Monoisotopic Mass | 32.037 g/mol |
|---|
| CAS Registry Number | 302-01-2 |
|---|
| IUPAC Name | hydrazine |
|---|
| Traditional Name | hydrazine |
|---|
| SMILES | NN |
|---|
| InChI Identifier | InChI=1S/H4N2/c1-2/h1-2H2 |
|---|
| InChI Key | InChIKey=OAKJQQAXSVQMHS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous other non-metal compounds. These are inorganic non-metallic compounds in which the largest atom belongs to the class of 'other non-metals'. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Homogeneous other non-metal compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous other non-metal compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Homogeneous other non metal
- Hydrazine derivative
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 2°C | | Boiling Point | Not Available | | Solubility | 1000 mg/mL | | LogP | -2.07 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9000000000-7e6c9f4fe38a72218c48 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9000000000-401f7c59e214d2145c6b | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-401f7c59e214d2145c6b | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-401f7c59e214d2145c6b | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9000000000-d34c4424b02bfa4ea545 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-d34c4424b02bfa4ea545 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-d34c4424b02bfa4ea545 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9000000000-45c21befbfc7d627145c | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-45c21befbfc7d627145c | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-45c21befbfc7d627145c | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9000000000-b155ef0ff565a5a1d372 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-b155ef0ff565a5a1d372 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-b155ef0ff565a5a1d372 | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (5); inhalation (5) ; dermal (5) |
|---|
| Mechanism of Toxicity | At least two mechanisms of action have been observed. One involves the direct binding of those hydrazines with a free amino group (hydrazine and 1,1-dimethylhydrazine) to key cellular molecules. Hydrazine reacts with alpha-keto acids such as vitamin B6 to form hydrazoines compounds. By binding to keto acids and forming hydrazones, hydrazine inhibits oxygen consumption with mitochondrial substrates in vitro. A second mechanism involves the generation of reactive species such as free radical intermediates or methyldiazonium ions as a result of metabolism. (6) |
|---|
| Metabolism | Hydrazines are likely to be more rapidly absorbed into the blood after ingestion or exposure to the skin than after inhalation. Once in the blood, they are probably carried to all the tissues of the body. Soon after exposure, the levels of hydrazines in the tissues fall since they are metabolised in several products such as acetyl-, diacetylhydrazine, pyruvate hydrazone, urea, and acyclic compound (1,4,5,6-tetrahydro-6-oxo-3-pyridazine carboxylic acid). However, these metabolites interacts with some important proteins and might be harmful to the body. Some studies showed that metabolites and unchanged hydrazine leave the body within one day.
Small amounts can also be found in the expired air. (6, 1) |
|---|
| Toxicity Values | LD50: 60 mg/kg (Oral, Rat)
LD50: 91 mg/kg (Dermal, Rabbit)
LC50: 570 ppm over 4 hours (Inhalation, Rat) (7) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (3) |
|---|
| Uses/Sources | Hydrazine is mainly used as a foaming agent in preparing polymer foams, but significant applications also include its uses as a precursor to polymerization catalysts and pharmaceuticals. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Exposure may occur from breathing contaminated air in or near a facility that makes, processes, or uses hydrazines, eating fish contaminated with hydrazines, drinking or swimming in water that has been contaminated with hydrazines, or touching soil contaminated with hydrazines, such as near some military bases or hazardous waste sites. Breathing cigarette smoke indirectly or using tobacco products may expose to small amounts of hydrazine or 1,1-dimethylhydrazine. (6) |
|---|
| Minimum Risk Level | Intermediate Inhalation: 0.004 ppm (2) |
|---|
| Health Effects | Breathing hydrazines for short periods may cause coughing and irritation of the throat and lungs, convulsions, tremors, or seizures. Breathing hydrazines for long periods may cause liver and kidney damage, as well as serious effects on reproductive organs. Eating or drinking small amounts of hydrazines may cause nausea, vomiting, uncontrolled shaking, inflammation of the nerves, drowsiness, or coma. (6) |
|---|
| Symptoms | Hydrazine may cause corrosive burning sensations, confusion, convulsions, abdominal cramps, headache, unconsciousness, vomiting, weakness, shortness of breath, or sore throat and cough, depending on the route of exposure. (5) |
|---|
| Treatment | Induced emesis, gastric lavage, use of saline cathartics, or activated charcoal are commonly used to decrease the gastrointestinal absorption of hydrazines. In general, these treatments are most effective when used within a few hours after oral exposure. Following dermal or ocular exposures to hydrazines, all contaminated clothing should be removed, and contacted skin should be washed immediately with soap and water. Eyes that have come in contact with hydrazines should be flushed with copious amounts of water. Contact lenses should be removed prior to flushing with water. (6) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB12973 |
|---|
| PubChem Compound ID | 9321 |
|---|
| ChEMBL ID | CHEMBL1237174 |
|---|
| ChemSpider ID | 8960 |
|---|
| KEGG ID | C05361 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 15571 |
|---|
| BioCyc ID | 4-HYDROXYMETHYLPHENYLHYDRAZINE |
|---|
| CTD ID | C029424 |
|---|
| Stitch ID | Hydrazine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 713 |
|---|
| Wikipedia Link | hydrazine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Sanins SM, Timbrell JA, Elcombe C, Nicholson JK: Proton NMR spectroscopic studies on the metabolism and biochemical effects of hydrazine in vivo. Arch Toxicol. 1992;66(7):489-95. [1332653 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Hydrazine. Last Updated 1 June 2009. [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1995). International Chemical Safety Card for Hydrazine. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1997). Toxicological profile for hydrazine. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- The Physical and Theoretical Chemistry Laboratory of Oxford University (2008). Material Safety Data Sheet (MSDS) for hydrazine (anhydrous). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | Not Available |
|---|