You are using an unsupported browser. Please upgrade your browser to a newer version to get the best experience on Toxin, Toxin Target Database.
Diazepam (T3D2903)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:27:45 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:53 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2903 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Diazepam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Diazepam is a benzodiazepine with anticonvulsant, anxiolytic, sedative, muscle relaxant, and amnesic properties and a long duration of action. Its actions are mediated by enhancement of gamma-aminobutyric acid activity. It is used in the treatment of severe anxiety disorders, as a hypnotic in the short-term management of insomnia, as a sedative and premedicant, as an anticonvulsant, and in the management of alcohol withdrawal syndrome. (From Martindale, The Extra Pharmacopoeia, 30th ed, p589). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
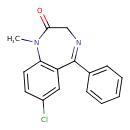
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C16H13ClN2O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 284.740 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 284.072 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 439-14-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 7-chloro-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | diazepam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CN1C2=C(C=C(Cl)C=C2)C(=NCC1=O)C1=CC=CC=C1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C16H13ClN2O/c1-19-14-8-7-12(17)9-13(14)16(18-10-15(19)20)11-5-3-2-4-6-11/h2-9H,10H2,1H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=AAOVKJBEBIDNHE-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as 1,4-benzodiazepines. These are organic compounds containing a benzene ring fused to a 1,4-azepine. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1,4-benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1,4-benzodiazepines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Parenteral (intramuscular); oral; enteral(rectal).Essentially complete, with a bioavailability of 93%. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Benzodiazepines bind nonspecifically to benzodiazepine receptors which mediate sleep, affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatic via the Cytochrome P450 enzyme system. The main active metabolite is desmethyldiazepam, in addition to minor active metabolites including temazepam and oxazepam. Route of Elimination: Diazepam and its metabolites are excreted mainly in the urine, predominantly as their glucuronide conjugates. Half Life: Biphasic 1-2 days and 2-5 days, active metabolites with long half lives. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 1200 mg/kg (oral, rat) (7) LD50: 1000 mg/kg (oral, dog) (7) LD50: 700 mg/kg (oral, mice) (7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Used in the treatment of severe anxiety disorders, as a hypnotic in the short-term management of insomnia, as a sedative and premedicant, as an anticonvulsant, and in the management of alcohol withdrawal syndrome. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. May cause a potentially dangerous rash that may develop into Stevens Johnson syndrome, an extremely rare but potentially fatal skin disease. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of overdose include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of norepinephrine or metaraminol. Dialysis is of limited value. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00829 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB14967 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 3016 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 2908 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C06948 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 49575 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Diazepam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | DZP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Diazepam | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Chase, G.; U.S. Patent 3,102,116; August 27, 1963; assigned to Hoffmann-La Roche Inc. Reeder, E. and Sternbach, L.H.; U.S. Patent 3,109,843; November 5, 1963; assigned to | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.011 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.0112 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.013 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.014 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.0143 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.015 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.033 uM | Not Available | BindingDB 50000766 |
| IC50 | 0.017 uM | Not Available | BindingDB 50000766 |
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, Swain NA: Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem. 2013 Feb 14;56(3):593-624. doi: 10.1021/jm3011433. Epub 2012 Nov 29. [23121096 ]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM: Pharmacophore/receptor models for GABA(A)/BzR subtypes (alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2) via a comprehensive ligand-mapping approach. J Med Chem. 2000 Jan 13;43(1):71-95. [10633039 ]
- Carling RW, Moore KW, Street LJ, Wild D, Isted C, Leeson PD, Thomas S, O'Connor D, McKernan RM, Quirk K, Cook SM, Atack JR, Wafford KA, Thompson SA, Dawson GR, Ferris P, Castro JL: 3-phenyl-6-(2-pyridyl)methyloxy-1,2,4-triazolo[3,4-a]phthalazines and analogues: high-affinity gamma-aminobutyric acid-A benzodiazepine receptor ligands with alpha 2, alpha 3, and alpha 5-subtype binding selectivity over alpha 1. J Med Chem. 2004 Mar 25;47(7):1807-22. [15027873 ]
- Russell MG, Carling RW, Atack JR, Bromidge FA, Cook SM, Hunt P, Isted C, Lucas M, McKernan RM, Mitchinson A, Moore KW, Narquizian R, Macaulay AJ, Thomas D, Thompson SA, Wafford KA, Castro JL: Discovery of functionally selective 7,8,9,10-tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-a]phthalazines as GABA A receptor agonists at the alpha3 subunit. J Med Chem. 2005 Mar 10;48(5):1367-83. [15743180 ]
- Goodacre SC, Street LJ, Hallett DJ, Crawforth JM, Kelly S, Owens AP, Blackaby WP, Lewis RT, Stanley J, Smith AJ, Ferris P, Sohal B, Cook SM, Pike A, Brown N, Wafford KA, Marshall G, Castro JL, Atack JR: Imidazo[1,2-a]pyrimidines as functionally selective and orally bioavailable GABA(A)alpha2/alpha3 binding site agonists for the treatment of anxiety disorders. J Med Chem. 2006 Jan 12;49(1):35-8. [16392789 ]
- Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, McKernan RM, Sohal B, Cook S, Pike A, Dawson GR, Bromidge FA, Wafford KA, Seabrook GR, Thompson SA, Marshall G, Pillai GV, Castro JL, Atack JR, MacLeod AM: Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazine s and analogues as subtype-selective inverse agonists for the GABA(A)alpha5 benzodiazepine binding site. J Med Chem. 2004 Jul 1;47(14):3642-57. [15214791 ]
- Russell MG, Carling RW, Street LJ, Hallett DJ, Goodacre S, Mezzogori E, Reader M, Cook SM, Bromidge FA, Newman R, Smith AJ, Wafford KA, Marshall GR, Reynolds DS, Dias R, Ferris P, Stanley J, Lincoln R, Tye SJ, Sheppard WF, Sohal B, Pike A, Dominguez M, Atack JR, Castro JL: Discovery of imidazo[1,2-b][1,2,4]triazines as GABA(A) alpha2/3 subtype selective agonists for the treatment of anxiety. J Med Chem. 2006 Feb 23;49(4):1235-8. [16480260 ]
- Carling RW, Madin A, Guiblin A, Russell MG, Moore KW, Mitchinson A, Sohal B, Pike A, Cook SM, Ragan IC, McKernan RM, Quirk K, Ferris P, Marshall G, Thompson SA, Wafford KA, Dawson GR, Atack JR, Harrison T, Castro JL, Street LJ: 7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl) -1,2,4-triazolo[4,3-b]pyridazine: a functionally selective gamma-aminobutyric acid(A) (GABA(A)) alpha2/alpha3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models. J Med Chem. 2005 Nov 17;48(23):7089-92. [16279764 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0066 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.02 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.0262 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.033 uM | Not Available | BindingDB 50000766 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Collins I, Davey WB, Rowley M, Quirk K, Bromidge FA, McKernan RM, Thompson SA, Wafford KA: N-(indol-3-ylglyoxylyl)piperidines: high affinity agonists of human GABA-A receptors containing the alpha1 subunit. Bioorg Med Chem Lett. 2000 Jun 19;10(12):1381-4. [10890169 ]
- Russell MG, Carling RW, Atack JR, Bromidge FA, Cook SM, Hunt P, Isted C, Lucas M, McKernan RM, Mitchinson A, Moore KW, Narquizian R, Macaulay AJ, Thomas D, Thompson SA, Wafford KA, Castro JL: Discovery of functionally selective 7,8,9,10-tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-a]phthalazines as GABA A receptor agonists at the alpha3 subunit. J Med Chem. 2005 Mar 10;48(5):1367-83. [15743180 ]
- Goodacre SC, Street LJ, Hallett DJ, Crawforth JM, Kelly S, Owens AP, Blackaby WP, Lewis RT, Stanley J, Smith AJ, Ferris P, Sohal B, Cook SM, Pike A, Brown N, Wafford KA, Marshall G, Castro JL, Atack JR: Imidazo[1,2-a]pyrimidines as functionally selective and orally bioavailable GABA(A)alpha2/alpha3 binding site agonists for the treatment of anxiety disorders. J Med Chem. 2006 Jan 12;49(1):35-8. [16392789 ]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM: Pharmacophore/receptor models for GABA(A)/BzR subtypes (alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2) via a comprehensive ligand-mapping approach. J Med Chem. 2000 Jan 13;43(1):71-95. [10633039 ]
- Carling RW, Moore KW, Street LJ, Wild D, Isted C, Leeson PD, Thomas S, O'Connor D, McKernan RM, Quirk K, Cook SM, Atack JR, Wafford KA, Thompson SA, Dawson GR, Ferris P, Castro JL: 3-phenyl-6-(2-pyridyl)methyloxy-1,2,4-triazolo[3,4-a]phthalazines and analogues: high-affinity gamma-aminobutyric acid-A benzodiazepine receptor ligands with alpha 2, alpha 3, and alpha 5-subtype binding selectivity over alpha 1. J Med Chem. 2004 Mar 25;47(7):1807-22. [15027873 ]
- Carling RW, Madin A, Guiblin A, Russell MG, Moore KW, Mitchinson A, Sohal B, Pike A, Cook SM, Ragan IC, McKernan RM, Quirk K, Ferris P, Marshall G, Thompson SA, Wafford KA, Dawson GR, Atack JR, Harrison T, Castro JL, Street LJ: 7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl) -1,2,4-triazolo[4,3-b]pyridazine: a functionally selective gamma-aminobutyric acid(A) (GABA(A)) alpha2/alpha3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models. J Med Chem. 2005 Nov 17;48(23):7089-92. [16279764 ]
- Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, McKernan RM, Sohal B, Cook S, Pike A, Dawson GR, Bromidge FA, Wafford KA, Seabrook GR, Thompson SA, Marshall G, Pillai GV, Castro JL, Atack JR, MacLeod AM: Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazine s and analogues as subtype-selective inverse agonists for the GABA(A)alpha5 benzodiazepine binding site. J Med Chem. 2004 Jul 1;47(14):3642-57. [15214791 ]
- Russell MG, Carling RW, Street LJ, Hallett DJ, Goodacre S, Mezzogori E, Reader M, Cook SM, Bromidge FA, Newman R, Smith AJ, Wafford KA, Marshall GR, Reynolds DS, Dias R, Ferris P, Stanley J, Lincoln R, Tye SJ, Sheppard WF, Sohal B, Pike A, Dominguez M, Atack JR, Castro JL: Discovery of imidazo[1,2-b][1,2,4]triazines as GABA(A) alpha2/3 subtype selective agonists for the treatment of anxiety. J Med Chem. 2006 Feb 23;49(4):1235-8. [16480260 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.01 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.014 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.015 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.0237 uM | Not Available | BindingDB 50000766 |
| Inhibitory | 0.033 uM | Not Available | BindingDB 50000766 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Jacobsen EJ, TenBrink RE, Stelzer LS, Belonga KL, Carter DB, Im HK, Im WB, Sethy VH, Tang AH, VonVoigtlander PF, Petke JD: High-affinity partial agonist imidazo[1,5-a]quinoxaline amides, carbamates, and ureas at the gamma-aminobutyric acid A/benzodiazepine receptor complex. J Med Chem. 1996 Jan 5;39(1):158-75. [8568803 ]
- Lager E, Andersson P, Nilsson J, Pettersson I, Nielsen EO, Nielsen M, Sterner O, Liljefors T: 4-quinolone derivatives: high-affinity ligands at the benzodiazepine site of brain GABA A receptors. synthesis, pharmacology, and pharmacophore modeling. J Med Chem. 2006 Apr 20;49(8):2526-33. [16610795 ]
- Carling RW, Moore KW, Street LJ, Wild D, Isted C, Leeson PD, Thomas S, O'Connor D, McKernan RM, Quirk K, Cook SM, Atack JR, Wafford KA, Thompson SA, Dawson GR, Ferris P, Castro JL: 3-phenyl-6-(2-pyridyl)methyloxy-1,2,4-triazolo[3,4-a]phthalazines and analogues: high-affinity gamma-aminobutyric acid-A benzodiazepine receptor ligands with alpha 2, alpha 3, and alpha 5-subtype binding selectivity over alpha 1. J Med Chem. 2004 Mar 25;47(7):1807-22. [15027873 ]
- Carling RW, Madin A, Guiblin A, Russell MG, Moore KW, Mitchinson A, Sohal B, Pike A, Cook SM, Ragan IC, McKernan RM, Quirk K, Ferris P, Marshall G, Thompson SA, Wafford KA, Dawson GR, Atack JR, Harrison T, Castro JL, Street LJ: 7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl) -1,2,4-triazolo[4,3-b]pyridazine: a functionally selective gamma-aminobutyric acid(A) (GABA(A)) alpha2/alpha3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models. J Med Chem. 2005 Nov 17;48(23):7089-92. [16279764 ]
- Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, McKernan RM, Sohal B, Cook S, Pike A, Dawson GR, Bromidge FA, Wafford KA, Seabrook GR, Thompson SA, Marshall G, Pillai GV, Castro JL, Atack JR, MacLeod AM: Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazine s and analogues as subtype-selective inverse agonists for the GABA(A)alpha5 benzodiazepine binding site. J Med Chem. 2004 Jul 1;47(14):3642-57. [15214791 ]
- Collins I, Davey WB, Rowley M, Quirk K, Bromidge FA, McKernan RM, Thompson SA, Wafford KA: N-(indol-3-ylglyoxylyl)piperidines: high affinity agonists of human GABA-A receptors containing the alpha1 subunit. Bioorg Med Chem Lett. 2000 Jun 19;10(12):1381-4. [10890169 ]
- Russell MG, Carling RW, Atack JR, Bromidge FA, Cook SM, Hunt P, Isted C, Lucas M, McKernan RM, Mitchinson A, Moore KW, Narquizian R, Macaulay AJ, Thomas D, Thompson SA, Wafford KA, Castro JL: Discovery of functionally selective 7,8,9,10-tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-a]phthalazines as GABA A receptor agonists at the alpha3 subunit. J Med Chem. 2005 Mar 10;48(5):1367-83. [15743180 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0168 uM | Not Available | BindingDB 50000766 |
| Dissociation | 0.036 uM | Not Available | BindingDB 50000766 |
References
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- Harris D, Clayton T, Cook J, Sahbaie P, Halliwell RF, Furtmuller R, Huck S, Sieghart W, DeLorey TM: Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the alpha5 subunit. J Med Chem. 2008 Jul 10;51(13):3788-803. doi: 10.1021/jm701433b. Epub 2008 Jun 7. [18537233 ]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ: Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996 Feb;49(2):253-9. [8632757 ]
- General Function:
- Cholesterol binding
- Specific Function:
- Can bind protoporphyrin IX and may play a role in the transport of porphyrins and heme (By similarity). Promotes the transport of cholesterol across mitochondrial membranes and may play a role in lipid metabolism (PubMed:24814875), but its precise physiological role is controversial. It is apparently not required for steroid hormone biosynthesis. Was initially identified as peripheral-type benzodiazepine receptor; can also bind isoquinoline carboxamides (PubMed:1847678).
- Gene Name:
- TSPO
- Uniprot ID:
- P30536
- Molecular Weight:
- 18827.81 Da
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Falchi AM, Battetta B, Sanna F, Piludu M, Sogos V, Serra M, Melis M, Putzolu M, Diaz G: Intracellular cholesterol changes induced by translocator protein (18 kDa) TSPO/PBR ligands. Neuropharmacology. 2007 Aug;53(2):318-29. Epub 2007 Jun 2. [17631921 ]
- Han WQ, Wang LJ, Sun XY, Li JS: Treatment of bactericide wastewater by combined process chemical coagulation, electrochemical oxidation and membrane bioreactor. J Hazard Mater. 2008 Mar 1;151(2-3):306-15. Epub 2007 Jun 7. [17662522 ]
- Chen Z, Kong X: Study of Candida albicans vaginitis model in Kunming mice. J Huazhong Univ Sci Technolog Med Sci. 2007 Jun;27(3):307-10. [17641849 ]
- Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6. [17139284 ]
- Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. [17016423 ]
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Derry JM, Dunn SM, Davies M: Identification of a residue in the gamma-aminobutyric acid type A receptor alpha subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J Neurochem. 2004 Mar;88(6):1431-8. [15009644 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 50000766 |
| Inhibitory | >7 uM | Not Available | BindingDB 50000766 |
References
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ: Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol. 1996 Feb;49(2):253-9. [8632757 ]
- TenBrink RE, Im WB, Sethy VH, Tang AH, Carter DB: Antagonist, partial agonist, and full agonist imidazo[1,5-a]quinoxaline amides and carbamates acting through the GABAA/benzodiazepine receptor. J Med Chem. 1994 Mar 18;37(6):758-68. [8145225 ]
- Jacobsen EJ, TenBrink RE, Stelzer LS, Belonga KL, Carter DB, Im HK, Im WB, Sethy VH, Tang AH, VonVoigtlander PF, Petke JD: High-affinity partial agonist imidazo[1,5-a]quinoxaline amides, carbamates, and ureas at the gamma-aminobutyric acid A/benzodiazepine receptor complex. J Med Chem. 1996 Jan 5;39(1):158-75. [8568803 ]
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- Iorio LC, Barnett A, Billard W: Selective affinity of 1-N-trifluoroethyl benzodiazepines for cerebellar type 1 receptor sites. Life Sci. 1984 Jul 2;35(1):105-13. [6738302 ]
- Wamsley JK, Golden JS, Yamamura HI, Barnett A: Autoradiographic demonstration of the selectivity of two 1-N-trifluoroethyl benzodiazepines for the BZD-1 receptors in the rat brain. Pharmacol Biochem Behav. 1985 Dec;23(6):973-8. [2867566 ]
- General Function:
- Transmembrane signaling receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRQ
- Uniprot ID:
- Q9UN88
- Molecular Weight:
- 72020.875 Da
References
- Mohler H, Fritschy JM, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002 Jan;300(1):2-8. [11752090 ]
- Riss J, Cloyd J, Gates J, Collins S: Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69-86. doi: 10.1111/j.1600-0404.2008.01004.x. Epub 2008 Mar 31. [18384456 ]
- General Function:
- G-protein coupled adenosine receptor activity
- Specific Function:
- Receptor for adenosine. The activity of this receptor is mediated by G proteins which inhibits adenylyl cyclase. Possible role in reproduction.
- Gene Name:
- ADORA3
- Uniprot ID:
- P0DMS8
- Molecular Weight:
- 36184.175 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.012 uM | Not Available | BindingDB 50000766 |
References
- Minetti P, Tinti MO, Carminati P, Castorina M, Di Cesare MA, Di Serio S, Gallo G, Ghirardi O, Giorgi F, Giorgi L, Piersanti G, Bartoccini F, Tarzia G: 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. J Med Chem. 2005 Nov 3;48(22):6887-96. [16250647 ]
- General Function:
- Peptide binding
- Specific Function:
- Receptor for cholecystokinin. Mediates pancreatic growth and enzyme secretion, smooth muscle contraction of the gall bladder and stomach. Has a 1000-fold higher affinity for CCK rather than for gastrin. It modulates feeding and dopamine-induced behavior in the central and peripheral nervous system. This receptor mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system.
- Gene Name:
- CCKAR
- Uniprot ID:
- P32238
- Molecular Weight:
- 47840.645 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >100 uM | Not Available | BindingDB 50000766 |
References
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Gould NP, Lundell GF, Homnick CF, Veber DF, et al.: Design of nonpeptidal ligands for a peptide receptor: cholecystokinin antagonists. J Med Chem. 1987 Jul;30(7):1229-39. [2885419 ]
- General Function:
- Not Available
- Specific Function:
- Keratin-binding protein required for epithelial cell polarization. Involved in apical junction complex (AJC) assembly via its interaction with PARD3. Required for ciliogenesis.
- Gene Name:
- FBF1
- Uniprot ID:
- Q8TES7
- Molecular Weight:
- 125445.19 Da
References
- Yoo MJ, Hage DS: Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J Chromatogr A. 2011 Apr 15;1218(15):2072-8. doi: 10.1016/j.chroma.2010.09.070. Epub 2010 Oct 16. [20956006 ]
- General Function:
- Toxic substance binding
- Specific Function:
- Serum albumin, the main protein of plasma, has a good binding capacity for water, Ca(2+), Na(+), K(+), fatty acids, hormones, bilirubin and drugs. Its main function is the regulation of the colloidal osmotic pressure of blood. Major zinc transporter in plasma, typically binds about 80% of all plasma zinc.
- Gene Name:
- ALB
- Uniprot ID:
- P02768
- Molecular Weight:
- 69365.94 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Dissociation | 24.547 uM | Not Available | BindingDB 50000766 |
References
- Lazaro E, Lowe PJ, Briand X, Faller B: New approach to measure protein binding based on a parallel artificial membrane assay and human serum albumin. J Med Chem. 2008 Apr 10;51(7):2009-17. doi: 10.1021/jm7012826. Epub 2008 Mar 19. [18348514 ]
24. GABA-A receptor (anion channel) (Protein Group)
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Included Proteins:
- P14867 , P47869 , P34903 , P48169 , P31644 , Q16445 , P18505 , P47870 , P28472 , O14764 , P78334 , Q8N1C3 , P18507 , Q99928 , O00591 , Q9UN88