| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-03 20:53:35 UTC |
|---|
| Update Date | 2014-12-24 20:25:31 UTC |
|---|
| Accession Number | T3D2452 |
|---|
| Identification |
|---|
| Common Name | (2S,4R,5S)-Muscarine |
|---|
| Class | Small Molecule |
|---|
| Description | Main toxic constituent of the fly fungus Amanita muscaria and various Inocybe species
(2S,4R,5S)-Muscarine belongs to the family of Oxolanes. These are organic compounds containing an oxolane (tetrahydrofuran) ring, which is a saturated aliphatic five-member ring containing one oxygen and five carbon atoms. |
|---|
| Compound Type | - Ether
- Food Toxin
- Fungal Toxin
- Metabolite
- Mycotoxin
- Natural Compound
- Organic Compound
|
|---|
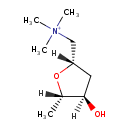
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+)-(2S,4R,5S)-Muscarine | | (+)-muscarine | | L-(+)-Muscarine | | Muscarin | | Muscarine | | Muscarine (alkaloid) | | Muscarine (the alkaloid) | | Muscarine chloride | | Muscarine II | | Muskarin | | Trimethyl(tetrahydro-4-hydroxy-5-methylfurfuryl)-Ammonium |
|
|---|
| Chemical Formula | C9H20NO2 |
|---|
| Average Molecular Mass | 174.260 g/mol |
|---|
| Monoisotopic Mass | 174.149 g/mol |
|---|
| CAS Registry Number | 300-54-9 |
|---|
| IUPAC Name | {[(2S,4R,5S)-4-hydroxy-5-methyloxolan-2-yl]methyl}trimethylazanium |
|---|
| Traditional Name | (+)-muscarine |
|---|
| SMILES | [H][C@@]1(C)O[C@]([H])(C[N+](C)(C)C)C[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C9H20NO2/c1-7-9(11)5-8(12-7)6-10(2,3)4/h7-9,11H,5-6H2,1-4H3/q+1/t7-,8-,9+/m0/s1 |
|---|
| InChI Key | InChIKey=UQOFGTXDASPNLL-XHNCKOQMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose monosaccharide
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Tetrahydrofuran
- Secondary alcohol
- Dialkyl ether
- Ether
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organonitrogen compound
- Amine
- Organic salt
- Alcohol
- Organic cation
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | Not Available | 2020-06-30 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-0f9y-6900000000-79763ab7b63262ffe2c2 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 10V, positive | splash10-00di-0900000000-c226ed17dd770fa6c411 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 15V, positive | splash10-00di-2900000000-67eb26beea6d618ebb7d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 17V, positive | splash10-00di-5900000000-4e0964583deb72b35ef6 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 20V, positive | splash10-05fr-9500000000-a0a0c909b980bb4769c2 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 23V, positive | splash10-0a4i-9200000000-be98c82a4035b83f8750 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 25V, positive | splash10-0a4i-9100000000-e8830a6e2498d5ca0b6f | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 27V, positive | splash10-0a4i-9000000000-19f49df6db6e4b92cf3d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 30V, positive | splash10-0a4l-9000000000-0755c01a97b2a22de481 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 33V, positive | splash10-0a4l-9000000000-b0b62b0c3630f7342703 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 35V, positive | splash10-052f-9000000000-0a2f7b3f7e5bf0ef5ed3 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QTOF 40V, positive | splash10-052f-9000000000-24069c492c822c8073ed | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, positive | splash10-00di-0900000000-e02b9884b570bdfc5d5c | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 6V, positive | splash10-00di-2900000000-1be2813c25bfaa5cb10c | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 9V, positive | splash10-05fr-9600000000-cb4c5b9dc39c97a63239 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 10V, positive | splash10-0c00-9100000000-2e0e0d8c33dd5405906b | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 13V, positive | splash10-0bt9-9000000000-1c66632fe3ec85e7e9b7 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 15V, positive | splash10-0bt9-9000000000-b821cc1b0c3b1409a2a4 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 19V, positive | splash10-0a4i-9000000000-4556237f549b235f4c6e | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 12V, positive | splash10-014i-4900000000-2e898bb2167bdd163be9 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 12V, positive | splash10-0a4i-9000000000-e58e4ee62799955c0939 | 2020-07-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0900000000-a0080208160655ea0b73 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-2900000000-9edf94eafe412073184c | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9100000000-c8c1131f5df11bfdcd19 | 2019-02-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (1) |
|---|
| Mechanism of Toxicity | Muscarine is a competitive inhibitor. It mimics the action of the neurotransmitter acetylcholine at acetylcholine receptors. (3) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Muscarine is only a trace compound in the fly agaric Amanita muscaria; the pharmacologically more relevant compound from this mushroom is muscimol.
Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. (3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Intoxication generally subsides within 2 hours. Death is rare, but may result from cardiac or respiratory failure in severe cases. (3) |
|---|
| Symptoms | Muscarine poisoning is characterized by increased salivation, sweating (perspiration), and tearflow (lacrimation) within 15 to 30 minutes after ingestion of the mushroom. With large doses, these symptoms may be followed by abdominal pain, severe nausea, diarrhea, blurred vision, and labored breathing. (3) |
|---|
| Treatment | The specific antidote is atropine. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB29936 |
|---|
| PubChem Compound ID | 5079496 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 4255959 |
|---|
| KEGG ID | C07473 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 522933 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D009116 |
|---|
| Stitch ID | Muscarine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Muscarine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D2452.pdf |
|---|
| General References | - Peraica M, Domijan AM: Contamination of food with mycotoxins and human health. Arh Hig Rada Toksikol. 2001 Mar;52(1):23-35. [11370295 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- Wikipedia. Muscarine. Last Updated 3 July 2009. [Link]
- Wikipedia. Mushroom poisoning. Last Updated 10 August 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|