| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:19 UTC |
|---|
| Update Date | 2014-12-24 20:21:22 UTC |
|---|
| Accession Number | T3D0229 |
|---|
| Identification |
|---|
| Common Name | Dimethoate |
|---|
| Class | Small Molecule |
|---|
| Description | Dimethoate is an organophosphate insecticide used to kill mites and insects systemically and on contact. It is used against a wide range of insects, including aphids, thrips, planthoppers and whiteflies on ornamental plants, alfalfa, apples, corn, cotton, grapefruit, grapes, lemons, melons, oranges, pears, pecans, safflower, sorghum, soybeans, tangerines, tobacco, tomatoes, watermelons, wheat and other vegetables. It is also used as a residual wall spray in farm buildings for house flies. Dimethoate has been administered to livestock for control of botflies. Dimethoate is moderately toxic and severe poisoning affects the central nervous system. (1) |
|---|
| Compound Type | - Amide
- Amine
- Ester
- Household Toxin
- Insecticide
- Organic Compound
- Organophosphate
- Pesticide
- Synthetic Compound
|
|---|
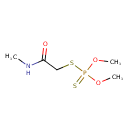
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-Dimethoxyphosphinothioylthio-N-methylacetamide | | Cygon | | Cygon 2-E | | Cygon 400 | | Cygon 4E | | Cygon insecticide | | Daphene | | De-fend | | Demos-L40 | | Devigon | | Dimate 267 | | Dimetate | | Dimethoate 30 EC | | Dimethoate solution | | Dimethogen | | Dimethoic acid | | Dimethyl S-((methylcarbamoyl)methyl) phosphorodithioate | | Dimethyl S-(N-(methylcarbamoyl)methyl) phosphorodithioate | | Dimeton | | Dimevur | | Ferkethion | | Fortion NM | | Fosfamid | | Fosfatox R | | Fosfotox | | Fosfotox R | | Fosfotox R 35 | | Fostion MM | | Lurgo | | Maxima phlanzenschutz | | O,O-dimethyl methylcarbamoylmethyl phosphorodithioate | | O,O-dimethyl S-((methylcarbamoyl)methyl)phosphorodithioate | | O,O-dimethyl S-(N-methylcarbamoylmethyl) dithiophosphate | | O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate | | O,O-dimethyl S-(N-methylcarbamylmethyl) thiothionophosphate | | O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate | | O,O-Dimethyl S-[2-(methylamino)-2-oxoethyl] dithiophosphate | | O,O-Dimethyl-S-(2-oxo-3-aza-butyl)-dithiophosphate | | Perfecthion | | Perfekthion | | Phosphamid | | Phosphamide | | Phosphorodithioic acid, O,O-dimethyl S-(2-(methylamino)-2-oxoethyl) ester | | Racusan | | Rebelate | | Rogodan | | Rogor | | Rogor 20L | | Rogor 40 | | Rogor l | | Rogor p | | Roxion | | Roxion ua | | S-methylcarbamoylmethyl O,O-dimethyl phosphorodithioate | | Sevigor | | Sinoratox | | Sistemin | | Solut | | Systemic insecticide | | Systemin | | Systoate | | Trimetion | | Turbair |
|
|---|
| Chemical Formula | C5H12NO3PS2 |
|---|
| Average Molecular Mass | 229.257 g/mol |
|---|
| Monoisotopic Mass | 229.000 g/mol |
|---|
| CAS Registry Number | 60-51-5 |
|---|
| IUPAC Name | O,O-dimethyl {[(methylcarbamoyl)methyl]sulfanyl}phosphonothioate |
|---|
| Traditional Name | dimethoate |
|---|
| SMILES | CNC(=O)CSP(=S)(OC)OC |
|---|
| InChI Identifier | InChI=1S/C5H12NO3PS2/c1-6-5(7)4-12-10(11,8-2)9-3/h4H2,1-3H3,(H,6,7) |
|---|
| InChI Key | InChIKey=MCWXGJITAZMZEV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dithiophosphate o-esters. These are o-ester derivatives of dithiophosphates, with the general structure RSP(O)(O)=S (R = organyl group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic dithiophosphoric acids and derivatives |
|---|
| Sub Class | Dithiophosphate O-esters |
|---|
| Direct Parent | Dithiophosphate O-esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dithiophosphate s-ester
- Dithiophosphate o-ester
- Carboxamide group
- Secondary carboxylic acid amide
- Sulfenyl compound
- Organothiophosphorus compound
- Carboxylic acid derivative
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Carbonyl group
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Dimethoate is a colorless crystalline solid with a camphor-like odor. (1) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 52°C | | Boiling Point | 117 °C (390°K) at 10 Pa | | Solubility | 25 mg/mL at 21 °C [MARTIN,H & WORTHING,CR (1977)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2020-08-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 10V, positive | splash10-0002-0910000000-ed8ec0dde52f74f3a67d | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 20V, positive | splash10-00di-0900000000-e025ead26ae3e68a1bd3 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 30V, positive | splash10-00di-0900000000-bef9184359bd74d3b461 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 40V, positive | splash10-00di-0900000000-3604a83f4707c27dddf7 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF 50V, positive | splash10-00di-0900000000-cf657743456c790d9583 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 16V, positive | splash10-0002-0900000000-a403532b2a700d0bb90e | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 6V, positive | splash10-0002-0920000000-d02ded0e6c91c275f535 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 13V, positive | splash10-00di-0900000000-dc425cd39054cc7d80ba | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 20V, positive | splash10-00dl-0900000000-6eae390613eea97ecbd1 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 27V, positive | splash10-00dl-1900000000-2f6bff3bd95d96c32e1b | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 34V, positive | splash10-006x-2900000000-0ee34ba6c3832a6b2d91 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 41V, positive | splash10-006x-4900000000-bf8f93d91440e270a87c | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 25V, positive | splash10-00di-0900000000-76eec21ed353331cbc72 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 36V, positive | splash10-00dl-2900000000-9c36234a485bc6baec04 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT 16V, positive | splash10-0002-0900000000-92b5ec6da125b0d970a1 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-00di-2900000000-efcc4129b0b2ce499949 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT 16V, positive | splash10-006y-1900000000-a64e8fc263dddfcc8028 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-00dl-0900000000-d43550cbd5a4aecf2907 | 2020-08-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-0udi-0390000000-612adcf5756794a5816a | 2020-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000t-2920000000-008d4aec36ec4daa873a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00e9-5940000000-cac4e89879d5ca21a230 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9200000000-f5ef08d5b522ad4f8406 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-056s-0970000000-7ecf0622179fb1cab244 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0731-1930000000-e6b9b176d6f431c404a3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01b9-2900000000-565c86ecd4f189da1096 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002u-9200000000-cf18e41a937e5d76b828 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100.40 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (1) ; inhalation (1) ; dermal (1) |
|---|
| Mechanism of Toxicity | Dimethoate is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. |
|---|
| Metabolism | Metabolism of organophosphates occurs principally by oxidation, by hydrolysis via esterases and by reaction with glutathione. Demethylation and glucuronidation may also occur. Oxidation of organophosphorus pesticides may result in moderately toxic products. In general, phosphorothioates are not directly toxic but require oxidative metabolism to the proximal toxin. The glutathione transferase reactions produce products that are, in most cases, of low toxicity. Paraoxonase (PON1) is a key enzyme in the metabolism of organophosphates. PON1 can inactivate some organophosphates through hydrolysis. PON1 hydrolyzes the active metabolites in several organophosphates insecticides as well as, nerve agents such as soman, sarin, and VX. The presence of PON1 polymorphisms causes there to be different enzyme levels and catalytic efficiency of this esterase, which in turn suggests that different individuals may be more susceptible to the toxic effect of organophosphate exposure. |
|---|
| Toxicity Values | LD50: 60 to 387 mg/kg (Oral, Rat)
LD50: 60 mg/kg (Oral, Mouse)
LD50: 400 mg/kg (Oral, Dog)
LD50: 200 mg/kg (Oral, Hamster)
LD50: 300 mg/kg (Oral, Rabbit)
LD50: 350 mg/kg (Oral, Guinea pig)
LD50: 100 mg/kg (Oral, Cat)
LD50: 1000 mg/kg (Dermal, Rabbit)
LD50: 353 mg/kg (Dermal, Rat)
LC50: 1.2 mg/l (Rat) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Spraying and application of nonarsenical insecticides entail exposures that are probably carcinogenic to humans (Group 2A). (2) |
|---|
| Uses/Sources | Dimethoate is an organophosphate insecticide used to kill mites and insects systemically and on contact. It is used against a wide range of insects, including aphids, thrips, planthoppers and whiteflies on ornamental plants, alfalfa, apples, corn, cotton, grapefruit, grapes, lemons, melons, oranges, pears, pecans, safflower, sorghum, soybeans, tangerines, tobacco, tomatoes, watermelons, wheat and other vegetables. It is also used as a residual wall spray in farm buildings for house flies. Dimethoate has been administered to livestock for control of botflies. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. |
|---|
| Symptoms | The first symptoms include bloody or runny nose, coughing, chest discomfort, difficult or short breath, and wheezing due to constriction or excess fluid in the bronchial tubes. Skin contact may cause localized sweating and involuntary muscle contractions. Eye contact will cause pain, bleeding, tears, pupil constriction, and blurred vision. Other symptoms following any way of exposure may include pallor, nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, eye pain, blurred vision, constriction or dilation of the eye pupils, tears, salivation, sweating, and confusion. Severe poisoning will affect the central nervous system, producing incoordination, slurred speech, loss of reflexes, weakness, fatigue, involuntary muscle contractions, twitching, tremors of the tongue or eyelids, and eventually paralysis of the body extremities and the respiratory muscles. In severe cases there may also be involuntary defecation or urination, psychosis, irregular heart beats, unconsciousness, convulsions and coma. Death may be caused by respiratory failure or cardiac arrest. (1)
|
|---|
| Treatment | If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 3082 |
|---|
| ChEMBL ID | CHEMBL1569524 |
|---|
| ChemSpider ID | 2973 |
|---|
| KEGG ID | C14326 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 34714 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D004117 |
|---|
| Stitch ID | Dimethoate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 487 |
|---|
| Wikipedia Link | Dimethoate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0229.pdf |
|---|
| General References | - Extension Toxicology Network (1993). Pesticide Information Profile for Dimethoate. A Pesticide Information Project of Cooperative Extension Offices of Cornell University, Michigan State University, Oregon State University, and University of California at Davis. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|