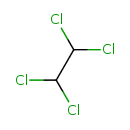
1,1,2,2-Tetrachloroethane (T3D0146)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-03-06 18:58:10 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:21:13 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D0146 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | 1,1,2,2-Tetrachloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 1,1,2,2-Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon. As a refrigerant, it is used under the name R-130. It was once widely used as a solvent and as an intermediate in the industrial production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene. 1,1,2,2-Tetrachloroethane was also used to separate fats and oils from other substances, to clean and degrease metals, and in paints and pesticides. However, 1,1,2,2-tetrachloroethane is no longer used much in the United States due to concerns about its toxicity. Less toxic chemicals are now available to replace this solvent, and largescale commercial production has stopped, although some production still occurs (5, 6). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C2H2Cl4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 167.849 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 165.891 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 79-34-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 1,1,2,2-tetrachloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | 1,1,2,2-tetrachloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | ClC(Cl)C(Cl)Cl | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C2H2Cl4/c3-1(4)2(5)6/h1-2H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=QPFMBZIOSGYJDE-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as organochlorides. Organochlorides are compounds containing a chemical bond between a carbon atom and a chlorine atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organohalogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organochlorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organochlorides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (7) ; inhalation (7) ; eye contact (7) ; dermal contact (7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | The presence of the functional group consisting of a terminal dichloromethyl moiety in a molecule is known to confer toxicity. Moreover, the metabolism of 1,1,2,2-tetrachloroethane to reactive products is also likely to play a key role in its toxicity. Both nuclear and microsomal cytochrome P450 enzymes (of the CYPIIA, CYPIIB, CYPIIE, and CYPIIIA subfamilies) have been implicated in the metabolism of the compound, possibly releasing a number of biologically active compounds, including aldehydes, alkenes, acids, and free radicals that may react with biological tissues. In general, the highly lipophilic nature of chlorinated hydrocarbons, such as 1,1,2,2-tetrachloroethane, allows them to cross the blood-brain barrier readily and partition into lipids in neuronal membranes. This property allows them to interfere with neural membrane function, bringing about central nervous system depression, behavioral changes, and anesthesia. 1,1,2,2-Tetrachloroethane has been shown to bind to DNA in the liver and several other organs, indicating that this mechanism may contribute to the carcinogenic process. Several studies of 1,1,2,2-tetrachloroethane toxicity have reported increases in the number of hepatocytes in mitosis, but the possible role these effects may have on the carcinogenicity of 1,1,2,2-tetrachloroethane has not been evaluated. (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | 1,1,2,2-tetrachloroethane appears to be distributed throughout the body, but may selectively accumulate to a degree in certain cells and tissues. Because it is a volatile, lipophilic molecule of small molecular size that appears to be readily absorbed from the respiratory and gastrointestinal tracts, passive diffusion is the most likely mechanism of absorption. The metabolism of 1,1,2,2-tetrachloroethane proceeds via multiple pathways. The predominant pathway appears to involve production of dichloroacetic acid, formed as an initial metabolite via stagewise hydrolytic cleavage of 1,1,2,2-tetrachloroethane, or by cytochrome P450-based oxidation of 1,1,2,2-tetrachloroethane. Dichloroacetic acid has been identified as the major urinary metabolite. Dichloroacetic acid can be further metabolized to glyoxylic acid, formic acid, and carbon dioxide, with carbon dioxide a potential major component of the end products. Other pathways involve the formation of trichloroethylene or tetrachloroethylene as initial metabolites, with subsequent reactions yielding trichloroethanol, trichloroacetic acid, and oxalic acid as important end products. Metabolism of 1,1,2,2-tetrachloroethane is generally extensive, with 68% or more of a total administered dose found as metabolites. Following absorption into the body, 1,1,2,2-tetrachloroethane is eliminated mainly as metabolites in the urine and as carbon dioxide and unchanged compound in expired air. (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 250 mg/kg/day (Oral, Rat) (5) LD50: 6360 mg/kg (Dermal, Rabbit) (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | 1,1,2,2-Tetrachloroethane was used as a refrigerant, a solvent, and as an intermediate in the industrial production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene. 1,1,2,2-Tetrachloroethane was also used to separate fats and oils from other substances, to clean and degrease metals, and in paints and pesticides. Exposure may occur from breathing concentrated fumes of 1,1,2,2-tetrachloroethane, drinking contaminated water or eating contaminated food, or through skin or eye contact. (5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Intermediate Oral: 0.5 mg/kg/day (Rat) (3, 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Liver, gastro-intestinal tract and nervous system (central peripheral) are the targets in chronic exposure. Toxic effects are also reported in the hematopoietic system. Breathing high levels of 1,1,2,2-tetrachloroethane for a long time can cause liver damage. Drinking very large amounts of 1,1,2,2-tetrachloroethane can cause shallow breathing, faint pulse, decreased blood pressure, and possibly unconsciousness. Central nervous system effects include general anesthesia, somnolence, hallucinations, and distorted perceptions. Other effects include narcosis, acute yellow atrophy of the liver, liver cirrhosis, fatty degeneration of the kidneys and heart, brain changes, changes in the peripheral nerves, hematemesis, purpuric rashes, and blood changes, including an increase in mononuclear leukocytes, progressive anemia, and slight thrombocytosis, hemolysis, salivation, restlessness, dizziness, nausea, vomiting, coma, and death. Monocytosis, dermatitis, liver tenderness and damage, delirium and convulsions may occur. Oliguria, cyanosis, uremia, peripheral paresthesia, and hypesthesia may also occur. (5, 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Abdominal pain, cough, sore throat, headache, nausea, vomiting, dizziness, drowsiness, confusion, tremor and convulsions can occur following inhalation, ingestion, as well as dermal contact with 1,1,2,2-tetrachloroethane. Redness and dry skin can follow dermal exposure. Eye exposure can lead to redness and pain of the eye(s). Exposure may also cause prickling sensation and numbness of limbs, loss of kneejerk, an unpleasant taste in the mouth, sweating, a deep dusky coloration of the skin, weight loss, pain over the liver, dark urine, and bilirubinuria. (7, 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | There is no specific treatment for trichloroethane poisoning. Gastric lavage is recommended after oral exposure. Protect airway by placement in Trendelenburg and left lateral decubitus position or by endotracheal intubation. Control any seizures first. In case of seizures following ingestion, administer a benzodiazepine IV. Following inhalation, move patient to fresh air. Monitor for respiratory distress. If cough or difficulty breathing develops, evaluate for respiratory tract irritation, bronchitis, or pneumonitis. Administer oxygen and assist ventilation as required. Treat bronchospasm with inhaled beta2 agonist and oral or parenteral corticosteroids. After eye exposure, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. If the exposure occurs through dermal contact, remove contaminated clothing and wash exposed area thoroughly with soap and water. In any case, a physician may need to examine the area if irritation or pain persists. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 6591 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL47258 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 6342 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C19534 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 36026 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | CPD-8985 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | C015530 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | 1,1,2,2-Tetrachloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | 1349 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | 1,1,2,2-Tetrachloroethane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | T3D0146.pdf | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Ligand-dependent nuclear transactivation involves either direct homodimer binding to a palindromic estrogen response element (ERE) sequence or association with other DNA-binding transcription factors, such as AP-1/c-Jun, c-Fos, ATF-2, Sp1 and Sp3, to mediate ERE-independent signaling. Ligand binding induces a conformational change allowing subsequent or combinatorial association with multiprotein coactivator complexes through LXXLL motifs of their respective components. Mutual transrepression occurs between the estrogen receptor (ER) and NF-kappa-B in a cell-type specific manner. Decreases NF-kappa-B DNA-binding activity and inhibits NF-kappa-B-mediated transcription from the IL6 promoter and displace RELA/p65 and associated coregulators from the promoter. Recruited to the NF-kappa-B response element of the CCL2 and IL8 promoters and can displace CREBBP. Present with NF-kappa-B components RELA/p65 and NFKB1/p50 on ERE sequences. Can also act synergistically with NF-kappa-B to activate transcription involving respective recruitment adjacent response elements; the function involves CREBBP. Can activate the transcriptional activity of TFF1. Also mediates membrane-initiated estrogen signaling involving various kinase cascades. Isoform 3 is involved in activation of NOS3 and endothelial nitric oxide production. Isoforms lacking one or several functional domains are thought to modulate transcriptional activity by competitive ligand or DNA binding and/or heterodimerization with the full length receptor. Essential for MTA1-mediated transcriptional regulation of BRCA1 and BCAS3. Isoform 3 can bind to ERE and inhibit isoform 1.
- Gene Name:
- ESR1
- Uniprot ID:
- P03372
- Molecular Weight:
- 66215.45 Da
References
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M: N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4. [16531984 ]
- Luft S, Milki E, Glustrom E, Ampiah-Bonney R, O'Hara P. Binding of Organochloride and Pyrethroid Pesticides To Estrogen Receptors α and β: A Fluorescence Polarization Assay. Biophysical Journal 2009;96(3):444a.
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. Binds estrogens with an affinity similar to that of ESR1, and activates expression of reporter genes containing estrogen response elements (ERE) in an estrogen-dependent manner (PubMed:20074560). Isoform beta-cx lacks ligand binding ability and has no or only very low ere binding activity resulting in the loss of ligand-dependent transactivation ability. DNA-binding by ESR1 and ESR2 is rapidly lost at 37 degrees Celsius in the absence of ligand while in the presence of 17 beta-estradiol and 4-hydroxy-tamoxifen loss in DNA-binding at elevated temperature is more gradual.
- Gene Name:
- ESR2
- Uniprot ID:
- Q92731
- Molecular Weight:
- 59215.765 Da
References
- Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M: N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006 Apr;69(8):1450-4. [16531984 ]
- Luft S, Milki E, Glustrom E, Ampiah-Bonney R, O'Hara P. Binding of Organochloride and Pyrethroid Pesticides To Estrogen Receptors α and β: A Fluorescence Polarization Assay. Biophysical Journal 2009;96(3):444a.
- General Function:
- Signal transducer activity
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of the calcium.
- Gene Name:
- ATP2C1
- Uniprot ID:
- P98194
- Molecular Weight:
- 100576.42 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium.
- Gene Name:
- ATP2C2
- Uniprot ID:
- O75185
- Molecular Weight:
- 103186.475 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
5. DNA
- General Function:
- Used for biological information storage.
- Specific Function:
- DNA contains the instructions needed for an organism to develop, survive and reproduce.
- Molecular Weight:
- 2.15 x 1012 Da
References
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for 1,1,2,2-tetrachloroethane. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA4
- Uniprot ID:
- P48169
- Molecular Weight:
- 61622.645 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transmembrane signaling receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRQ
- Uniprot ID:
- Q9UN88
- Molecular Weight:
- 72020.875 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B1
- Uniprot ID:
- P20020
- Molecular Weight:
- 138754.045 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Protein c-terminus binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B2
- Uniprot ID:
- Q01814
- Molecular Weight:
- 136875.18 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Pdz domain binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell.
- Gene Name:
- ATP2B3
- Uniprot ID:
- Q16720
- Molecular Weight:
- 134196.025 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Scaffold protein binding
- Specific Function:
- Calcium/calmodulin-regulated and magnesium-dependent enzyme that catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell (PubMed:8530416). By regulating sperm cell calcium homeostasis, may play a role in sperm motility (By similarity).
- Gene Name:
- ATP2B4
- Uniprot ID:
- P23634
- Molecular Weight:
- 137919.03 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Protein homodimerization activity
- Specific Function:
- Key regulator of striated muscle performance by acting as the major Ca(2+) ATPase responsible for the reuptake of cytosolic Ca(2+) into the sarcoplasmic reticulum. Catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A1
- Uniprot ID:
- O14983
- Molecular Weight:
- 110251.36 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- S100 protein binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Isoform 2 is involved in the regulation of the contraction/relaxation cycle.
- Gene Name:
- ATP2A2
- Uniprot ID:
- P16615
- Molecular Weight:
- 114755.765 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Metal ion binding
- Specific Function:
- This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium. Transports calcium ions from the cytosol into the sarcoplasmic/endoplasmic reticulum lumen. Contributes to calcium sequestration involved in muscular excitation/contraction.
- Gene Name:
- ATP2A3
- Uniprot ID:
- Q93084
- Molecular Weight:
- 113976.23 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A1
- Uniprot ID:
- P05023
- Molecular Weight:
- 112895.01 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A2
- Uniprot ID:
- P50993
- Molecular Weight:
- 112264.385 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Steroid hormone binding
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients.
- Gene Name:
- ATP1A3
- Uniprot ID:
- P13637
- Molecular Weight:
- 111747.51 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of sodium and potassium ions across the plasma membrane. This action creates the electrochemical gradient of sodium and potassium ions, providing the energy for active transport of various nutrients. Plays a role in sperm motility.
- Gene Name:
- ATP1A4
- Uniprot ID:
- Q13733
- Molecular Weight:
- 114165.44 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The beta subunit regulates, through assembly of alpha/beta heterodimers, the number of sodium pumps transported to the plasma membrane.Involved in cell adhesion and establishing epithelial cell polarity.
- Gene Name:
- ATP1B1
- Uniprot ID:
- P05026
- Molecular Weight:
- 35061.07 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-2 subunit is not known.Mediates cell adhesion of neurons and astrocytes, and promotes neurite outgrowth.
- Gene Name:
- ATP1B2
- Uniprot ID:
- P14415
- Molecular Weight:
- 33366.925 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Sodium:potassium-exchanging atpase activity
- Specific Function:
- This is the non-catalytic component of the active enzyme, which catalyzes the hydrolysis of ATP coupled with the exchange of Na(+) and K(+) ions across the plasma membrane. The exact function of the beta-3 subunit is not known.
- Gene Name:
- ATP1B3
- Uniprot ID:
- P54709
- Molecular Weight:
- 31512.34 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.
- General Function:
- Transporter activity
- Specific Function:
- May be involved in forming the receptor site for cardiac glycoside binding or may modulate the transport function of the sodium ATPase.
- Gene Name:
- FXYD2
- Uniprot ID:
- P54710
- Molecular Weight:
- 7283.265 Da
References
- Casarett LJ, Klaassen CD, and Watkins JB (2003). Casarett and Doull's essentials of toxicology. New York: McGraw-Hill/Medical Pub. Div.